por Kiko Ramos | May 29, 2025 | Equipment analysis
Regulation of medical devices is an essential pillar in ensuring patient safety, medical efficacy and diagnostic quality throughout the healthcare system. All medical devices must meet stringent legal and technical requirements before they can be marketed and used in clinical practice.
In this context, the application of different measures and regulations for the regulation of medical equipment aims to protect users. And, at the same time, to bring confidence to healthcare professionals, transparency to manufacturing processes and traceability throughout the supply chain.
However, it is important to note that certifying a medical equipment is not just another administrative procedure. It is a multidisciplinary process ranging from design and clinical validation to the implementation of quality systems, risk management and post-marketing surveillance.
But what are the different regulations and processes that must be applied to market and use medical devices in clinical practice? In the following article, we provide a complete guide to the regulation of medical equipment and the current legal frameworks, both at national, European and international level.
What are medical devices?
The medical equipment is devices, apparatus or systems used in the prevention, diagnosis, treatment or rehabilitation of diseases and medical conditions. They range from simple medical devices such as syringes or thermometers to complex technologies such as medical equipment, such as magnetic resonance imaging, ultrasound scannersremote monitoring systems or AI software of radiodiagnostics based on artificial intelligence.
Medical devices can be classified into different categories according to their level of risk for the patient and the user, which directly influences the type of regulation and certification that they must comply with.
Regulation of medical devices: The different regulations and processes
Regulation of medical devices varies from country to country or region to region, but in general, all medical devices are regulated in the United States. regulatory frameworks share a common goal: to ensure that the products are safe and effective for clinical use. Below, we review the different regulations and processes:
CE marking in the European context
In order to launch a medical device or medical equipment on the market and be marketed in the European Union, it must be CE marked. The CE marking is the legal requirement that authorizes the marketing of a medical device in the European Union (EU) and the European Economic Area (EEA).
Indicates that the product complies with the provisions of Regulation (EU) 2017/745 on medical devices.the so-called MDR regulationsand can circulate freely within the European market. This regulation came into use in 2017 and replaced the previous Directive 93/42/EEC with the aim of strengthening the safety, traceability and post-market control of devices.
The first step in determining the CE marking process is to carry out the classification of medical equipment according to risk. Depending on the type of riskthe MDR standard defines a level of technical documentation and a regulatory process to ensure access to the European market. Specifically, medical devices are divided into four classes:
- Class ILow risk
- Class IIaModerate risk
- Class IIbHigh risk
- Class IIIVery high risk. Use of critical implantable/invasive devices
What does CE marking imply?
The CE marking is not a seal of quality, but rather a legal compliance indicator at European level. It guarantees that the manufacturer has followed a series of processes that certify the safety and efficacy of the medical device:
- Evaluation of the device according to the legal requirements of MDR regulations.
- The use of the medical device is safe and effective when used as directed.
- Implementation of a adequate quality management system.
- Use of post-marketing surveillance mechanisms and continuous clinical follow-up.
- Assessment by a Notified Body.
FDA Approval in the United States
In the United Statesthe Food and Drug Administration (FDA) is the government agency responsible for the regulation, supervision and authorization of the marketing of medical devices.
It is developed through the Center for Devices and Radiological Health (CDRH)The FDA is an entity that ensures that all medical devices are safe, effective and manufactured according to quality standards before reaching the market. It classifies products into three categories:
- Class ILow risk, subject to general controls.
- Class IIModerate risk, require a 510(k) submission to demonstrate that they are substantially equivalent to another legally marketed device.
- Class IIIHigh risk. Require premarket approval (PMA) with detailed clinical evidence.
Key differences between FDA Approval and CE marking
| Appearance |
FDA (USA) |
EC (EU) |
| Scope |
United States and countries that recognize the FDA |
European Union and countries that accept CE marking |
| Target |
To authorize the marketing of medical devices under the direct supervision of the FDA. |
To certify that the product complies with the safety and performance requirements defined in the MDR Regulation. |
| Approach |
Stricter pre-market assessment |
Evaluation based on manufacturer's conformity |
| Clinical review |
PMA requires clinical trials |
Only Class III requires robust clinical evidence |
| Times |
Long, especially in PMA |
More agile in classes I and IIa |
| Evaluating Agency |
Public agency (FDA) |
Independent Notified Body |
Regulation of medical devices in Spain: The role of the AEMPS
At Spainthe Spanish Agency of Medicines and Health Products (AEMPS) is the body responsible for applying national and European regulations on medical devices. Its main mission is to guarantee the quality, safety, efficacy and correct information of all medical devices marketed or used in the country.
The AEMPS under the Ministry of Health and acts as the competent authority to supervise compliance with Regulation (EU) 2017/745 on medical devices (MDR), as well as other national provisions. In Spain, the legal framework on the regulation of medical devices is articulated in terms of the following. regulations:
- Royal Decree 192/2023, which regulates medical devicesIt is the most recent and complete national regulation on medical devices. It aims to adapt Regulation (EU) 2017/745 to the Spanish legal system.
- Law 29/2006, on guarantees and rational use of medicines and health products.This Law is the general legal framework for everything related to medicines and medical devices in Spain. Although it was born focused on pharmaceuticals, it has been modified and extended to include medical devices.
- Technical guides and procedures issued by the AEMPS itself.The AEMPS regularly issues technical documents, instructions and guides that help manufacturers and economic agents to correctly interpret and comply with the standards.
ISO standards: Standardization and quality control in medical devices
The ISO (International Organization for Standardization) standards are voluntary international standards that establish best practices for the design, production, evaluation and management of products and services.
In the medical devices field, are not a substitute for legislationsuch as MDR regulations in the European context or FDA approval in the United States. However, they are key instruments for demonstrate compliance with regulatory requirements and ensure the safety, efficacy and traceability of the product. The most relevant ISO standards are highlighted below:

ISO 13485:2016 - Quality management systems for medical devices.
It is the most important standard in this sector. It establishes the requirements for a quality management system (QMS) specifically tailored to medical device manufacturers and distributors.
Regulatory application
Not required by lawbut the implementation of an ISO 13485 system makes it easier to obtain CE markingIt reduces errors and demonstrates a commitment to quality and patient safety.
ISO 14971:2019 - Risk management for medical devices.
Establishes a structured framework for identifying, assessing, controlling and monitoring risk related to a medical device throughout its life cycle.
Regulatory application
It is mandatory in the context of the MDR to demonstrate that a thorough risk assessment has been performed. It is closely integrated with the technical documentation and clinical evaluation of the device.
ISO 10993 - Biological evaluation of medical devices
This set of standards addresses the biocompatible tests necessary to ensure that the materials in contact with the human body are non-toxic or provoke adverse reactions.
Regulatory application
These tests are mandatory for all devices that come into direct or indirect contact with the human body. Their compliance is part of the technical dossier submitted for CE marking or FDA approval.
ISO 14155: Human clinical trials of medical devices - Good clinical practice
Establishes the principles and requirements for the design, conduct, recording and presentation of clinical trials made in humans with medical devices.
Regulatory application
It is mandatory for manufacturers conducting prior clinical studies to CE or FDA certification, especially for class III or implantable devices.
ISO 62304: Software for medical devices - Software lifecycle processes
Defines the software development, maintenance and support requirements used as part of a medical device, or what constitutes a Software as a Medical Device (SaMD).
Regulatory application
As a result mandatory for any healthcare software that affects the diagnosis, treatment or monitoring of patients, either standalone or integrated in hardware. This is a key aspect in products such as medical apps, PACS systems or intelligent ultrasound scanners.
Conclusion
Patient safety starts with responsible certification. Regulatory applications are a key aspect of achieving responsible patient safety. safe and effective commercialization in the field of medical devices. Beyond a mere legal requirement, they represent a structured and rigorous process that ensures that medical devices have been designed, evaluated and manufactured under the most stringent quality standards. high standards of quality, safety and efficiency.
Whether under the CE marking framework in Europe, the FDA in the United States or national health authorities such as the AEMPS in Spain, evaluation and certification procedures are indispensable to ensure that technological innovation in the healthcare sector reaches the market in a responsible, efficient and sustainable manner.
Do you need medical equipment? In that case, do not hesitate to contact us and our 4D team will help you choose the best medical device according to the needs of your clinic or hospital.
Contact 4D
Kiko Ramos
CEO of 4D Médica. Expert in marketing and distribution of medical equipment.

por Luis Daniel Fernádez | May 21, 2025 | Equipment analysis
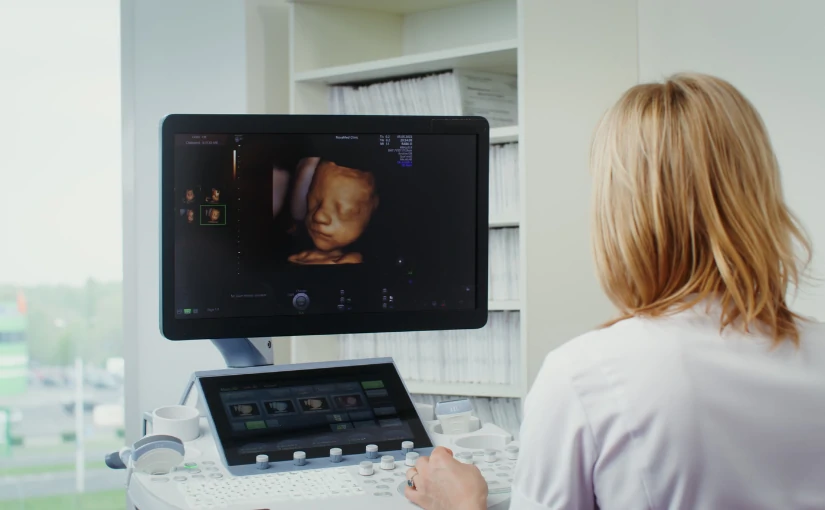
At present, the ultrasound scanner has established itself as a fundamental tool in the area of diagnostic imaging. This device uses the ultrasound technology to obtain accurate, real-time images of the internal structures of the human body, facilitating the evaluation of organs, tissues and blood vessels. without the need for invasive procedures.
The ability of ultrasound to provide detailed, safe and rapid information has revolutionized clinical practice. The use of this medical equipment enables healthcare professionals to detect and monitor a wide variety of pathologies in an early and effective manner. In addition, its versatility and portability has extended its use to multiple medical specialties.
The use of the ultrasound scanner is used to perform ultrasounds in order to analyze organs and tissues internally. It is one of the most widely used medical techniques, as it is a fast, effective and non-invasive method. It is mainly used to detect diseases and anomalies, to monitor the health of patients, to study the development and growth of the baby during pregnancy, as well as to guide certain medical procedures.
In contrast to other imaging techniques, such as X-rays or the Computed Axial Tomography (CT) scanultrasound does not use ionizing radiation, which makes it a safer technique. In addition, its portability and ease of use has enabled its integration in consultation rooms, emergency rooms and intensive care units, facilitating real-time clinical decision making and improving patient care.
In the following article, we analyze the origin of this medical equipment up to the present day, how an ultrasound scanner works, as well as its applications in clinical practice.
Origin of the ultrasound scanner: From its beginnings to the present day.
The development of the ultrasound scanner is closely linked to the development of ultrasound technology and its application in the medical field.

Early studies: Discovery of the piezoelectric effect
The first studies on ultrasonic waves date back to the end of the 19th century, when the French physicists Pierre and Jacques Curie discovered in 1880 the piezoelectric effect. This physical phenomenon consists of the ability of certain materials, such as quartz and some ceramic crystals, to generate an electrical charge when subjected to mechanical pressure.
The importance of the piezoelectric effect in ultrasound is fundamental, since it constitutes the basis of ultrasound transducer or probe operation. In practice, piezoelectric crystals located in the transducer convert electrical signals into ultrasonic vibrations (ultrasound waves), which are transmitted to the patient's body. Through the piezoelectric effect, these echoes are transformed into electrical signals that are processed by the ultrasound scanner to generate images in real time.
Development of the first ultrasound scanner: From the piezoelectric effect and ultrasound to the medical field
Following the discovery of the piezoelectric effect, the phenomenon was initially applied in industrial and military fields, particularly in the development of sonar devices for underwater detection during World War I and World War II.
However, the potential of ultrasound and the piezoelectric effect to generate and receive acoustic waves did not go unnoticed by the scientific and medical community. The adaptation of this technology to the medical field began in the middle of the 20th century. The Scottish physician Ian Donald, together with engineer Tom Brownwere the pioneering researchers who began to apply the piezoelectric effect to clinical explorationThe first of its kind, it laid the foundations of medical ultrasound.
Specifically, it was in the 1950s, when researchers developed the first clinical ultrasound prototype. Initially, ultrasound was used in obstetrics to visualize the fetus and detect pathologies during pregnancy, which was a revolution in prenatal monitoring.
From the 1960s and 1970s to the present day: Advances in ultrasonography
During the 1960s and 1970s, ultrasound technology made significant advances. It became from still images to real-time ultrasound scanswhich made it possible to observe the movement of internal organs and structures. Subsequently, the incorporation of the Doppler effect made possible the study of blood flow and vascular evaluation.The new ultrasound system has been designed to further expand the clinical applications of the ultrasound scanner.
In recent decades, the development of technology and information technology has made possible the emergence of more compact, portable ultrasound scanners with higher image resolution. Today, the ultrasound scanner is a safe, effective and versatile tool used in a wide variety of specialties, from emergency medicine to cardiology, gynecology and internal medicine. As a result, it has become an indispensable piece of medical equipment in medical practice.
Next-generation ultrasound scanners: innovation, technology and artificial intelligence
In recent years, technology has advanced greatly in the field of medicine. The state-of-the-art ultrasound scanners offer images in 3D, 4D and 5D technologyand therefore allow for visualize the inside of the human body in motion and in real time.
One of the most recent innovations is the ultrasound scanners that incorporate digital processing systems that apply the artificial intelligence in medical image analysis. The use of a AI software in ultrasound equipment provides greater speed, efficiency, safety and diagnostic accuracy, providing advanced and detailed analysis that improves clinical decision making.
In this area, it is worth mentioning the use of state-of-the-art ultrasound scanners to visualize the fetus in real time. It is known as emotional ultrasound and allows parents to get to know the baby before it is born. This type of ultrasound combines the 3D technologywhich provides three-dimensional images, with 4D and 5D technologyThe newborn's movements can be seen in real time with high image clarity and quality. With this, the baby's main movements can be seen. From yawning, opening the eyes and moving to changing position.
How an ultrasound scanner works: Step-by-step procedure
The ultrasound scanners are an essential tool in medical practice. Understand its operation and the workflow during an ultrasound examination is essential to ensure diagnostic quality and patient safety. Below, we discuss how an ultrasound scanner works and the step-by-step procedure:

1. Preparation of the patient and application of the conductive gel
First of all, the patient is instructed on the position The position to be used depends on the area to be scanned and the type of diagnosis to be made. Before starting the scan, the conductive gel on the patient's skin. It has the function of eliminating the air that is generated between the skin of the area to be examined and the transducer or ultrasound probe, facilitating the transmission of ultrasonic waves.
Selection of transducer type
One of the most important components of the ultrasound scanner is the transducer or probe. There are different types of transducersEach is designed to scan different regions and depths. While linear transducers are used for vascular and superficial studies, convex models are useful for deep abdominal scans.
Therefore, the medical professional will be in charge of selecting the type of transducer, connect it to the equipment and verify its correct operation. before starting the study.
3. Ultrasound emission and reception
Once the transducer is prepared, the operator places it on the gel-covered area. The transducer emits high-frequency ultrasound waves that penetrate the patient's internal tissues. When these waves pass through the body and are reflected at the interfaces of the different tissues and organs, the reflected waves, known as echoes, return to the transducer..
4. Echo pickup
The transducer also acts as a receiverby detecting the reflected waves (echoes) that are generated from the various internal structures. These echoes contain information on the location and characteristics of the traversed tissuesThis allows us to analyze the state and functioning of the different organs.
5. Image processing
Echoes picked up by the transducer are converted into electrical signals.These images are processed by the ultrasound console through different algorithms. The result is the generation of two-dimensional or three-dimensional images in real time that are displayed on the screen of the equipment.
By analyzing medical images, the operator is able to observe the anatomy and movement of internal organs and structures. In turn, with the use of the Doppler mode, the blood flow in the tissues can be studied.
6. Systematic exploration
The professional performs a methodical sweep by moving the transducer over the area of interest to be analyzedThe different sections (longitudinal, transversal, oblique) are obtained to fully examine the organs and structures. This systematic exploration is the key to obtain a complete and detailed diagnosisThe results of the study should be documented in a way that does not omit relevant findings, and the results should be adequately documented.
7. Adjustment of image parameters
During scanning, the operator can adjust various parametersThe display mode (2D, 3D, Doppler) can be set from the gain (brightness), depth and focus to the display mode (2D, 3D, Doppler). In this way, you can optimize image quality and adapt it to the anatomical characteristics of the patient.
8. Interpretation of medical images
After performing the examination, the physician is in charge of analyze the images obtained in real timeThe data can be used to identify possible alterations and to make static captures or recordings of relevant sequences. By means of these recordings, the final report can be fully documented, which will serve as the basis for the diagnosis and the clinical decision making.
9. Completion and cleaning
At the conclusion of the study, the operator removes the gel from the patient's skin. Subsequently, a protocol for disinfection and cleaning of the equipment usedThe transducer and the contact surface between each patient.
This structured process makes ultrasound a fast, safe, non-invasive and highly versatile technique, facilitating the accurate assessment of multiple organs and pathologies in daily clinical practice.
Main clinical applications of ultrasound
The use of ultrasound scanners covers practically all medical specialties. The main clinical applications include the following areas:
Obstetrics and gynecology
Ultrasound is essential in the pregnancy monitoringIt is used to evaluate fetal development, the location and viability of the pregnancy, the detection of congenital anomalies and the control of obstetric complications. It is also used for the study of gynecological pathologiesas ovarian cysts, uterine myomas or endometrial alterations.
Cardiology
The echocardiogram is an essential technique for the assessment of cardiac anatomy and functionallowing to diagnose valvular diseases, cardiomyopathies, heart failure, congenital heart diseases and evaluate blood flow using the Doppler mode.
Internal medicine and gastroenterology
The abdominal ultrasound allows examination of organs such as the liver, gallbladder, pancreas, kidneys, spleen and bladder. It therefore plays a key role in the diagnosis of masses, cysts, stones, inflammations and other pathologies. It is also used in the assessment of ascites and in the control of interventional procedures.
Vascular exploration
Through the Doppler ultrasoundthe blood flow in arteries and veinsIt is therefore used in the diagnosis of deep vein thrombosis, venous insufficiency, arterial stenosis, aneurysms and other vascular diseases.
Musculoskeletal
Allows you to study muscles, tendons, ligaments, joints and soft parts, facilitating the diagnosis of sports injuries, tears, tendinitis, bursitis, hemorrhages and subcutaneous masses.
Urology
It is used for assess the prostate, bladder, testicles and kidneysbeing useful in the diagnosis of prostatic hyperplasia, lithiasis, tumors and other urological alterations.
Pediatrics
Ultrasonography is especially useful in the study of pediatric pathologies, such as the hip dysplasia, hydrocephalus, renal malformations and abdominal alterations in newborns and infants.
Guidance on interventional procedures
The ultrasound scanner facilitates the performance of biopsies, drainages, punctures, catheter placement and other interventionsThe procedure is safer and more accurate.
Emergency medicine and intensive care
Its speed and portability allow immediate diagnosis of serious pathologies such as pleural effusions, hemoperitoneum, pneumothorax, cardiac tamponade and rapid assessment in polytraumatized patients (FAST ultrasound).
Conclusion
Ultrasound has established itself as an essential tool in clinical practice.It offers an accurate, efficient, safe and real-time medical analysis of the different internal organs and tissues. Its non-invasivenessThe absence of ionizing radiation and its versatility to be adapted to multiple specialties make it an indispensable resource both in the initial evaluation and in the follow-up of numerous pathologies.
Your portability and speed facilitate clinical decision making in a variety of settings, from outpatient to emergency situations. From its origins to the present day, the use of ultrasound has revolutionized medical practice by improving the quality of health care and making a decisive contribution to a early, accurate and safe diagnosis for patients.
If you want to obtain more information about ultrasound scanners or other medical diagnostic equipment, you can contact us. Our 4D team will give you advice to find the best solution for your clinic or hospital.
Contact 4D
Bibliography
García, J., & González, A. (2007). Ultrasound methodology and techniques. Physical principles and image formation. Medicina de Familia SEMERGEN, 33(2), 83-92. Retrieved May 20, 2025, from.
https://www.elsevier.es/es-revista-medicina-familia-semergen-40-articulo-metodologia-tecnicas-ecografia-principios-fisicos-13109445
Spanish Society of Pediatric Intensive Care (SECIP) (2018). Basic fundamentals of ultrasound. Retrieved May 20, 2025, from. https://secip.com/images/uploads/2018/09/1-FUNDAMENTOS-BASICOS-DE-ECOGRAF%C3%8DA.pdf
Authorea (n.d.). Ultrasound: Physical principles and clinical applications. Authorea. Retrieved May 20, 2025, from. https://www.authorea.com/doi/full/10.22541/au.172660489.98960333
Luis Daniel Fernandez Perez
Director of Diagximag. Distributor of medical imaging equipment and solutions.